Research Areas
Disease models in the Arditi Laboratory include cardiovascular inflammation, the role of innate and adaptive immunity in atherosclerosis and infection-induced acceleration of atherosclerosis in various hypercholesterolemic mouse models, Kawasaki disease vasculitis mouse model or coronary arteritis, aortitis, myocarditis and abdominal aorta aneurysm model.
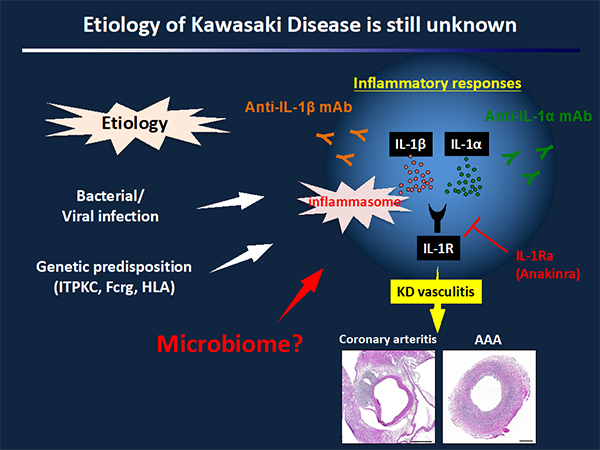
Kawasaki disease (KD) is an acute febrile illness and systemic vasculitis of unknown etiology that predominantly affects young children, causes coronary artery abnormalities and aneurysms, and could potentially result in long-term cardiovascular sequelae and even death. While intravenous immunoglobulin (IVIG) treatment lowers the risk of developing coronary artery aneurysms to 5%, up to 20% of KD patients are IVIG-resistant and are at greater risk for coronary artery aneurysms. Therefore, discovery of more effective treatments to prevent the cardiovascular complications of KD vasculitis is a high priority in pediatric and cardiovascular research.
The Lactobacillus casei cell-wall extract (LCWE) mouse model of KD vasculitis closely reproduces the important histologic as well as pathologic and immune features of the human disease. A single i.p. injection of LCWE into wild type mice reproducibly induces aortitis, proximal coronary arteritis, myocarditis as well as other systemic artery abnormalities such as abdominal aorta dilatation. By using the LCWE murine model of KD vasculitis, the Arditi Laboratory has found an absolute requirement for the NLRP3 inflammasome and IL-1β signaling. The lab has also discovered that anti-IL-1β treatment is more efficient than IVIG in preventing coronary lesion formation. These studies have led to multiple Phase II clinical trials to test Anakinra in IVIG-resistant KD patients.
The Arditi Lab has recently published the results of an open label phase II study that showed Anakinra is safe, well tolerated and may have efficacy in reducing fever and inflammation, and preventing coronary artery aneurysms.
The Arditi Laboratory research focus is on understanding molecular mechanisms of atherosclerosis and vascular inflammation. The lab is interested in the role of TLRs and innate immunity in hypercholesterolemic mouse models of atherosclerosis as well as the role of these innate immune receptors on Chlamydophila pneumoniae-induced acceleration of atherosclerosis. The Arditi Lab investigates the role of innate immunity, the role of NLRP3 inflammasome and IL-1, as well as IL-17 pathways in atherosclerosis. Recent focus of our research has been to investigate the role of autophagy and mitophagy and the role of mitochondrial oxidative DNA stress in atherosclerosis mouse models that also includes systemic lupus erythematosus mouse models. Gender differences in the differential role of NLRP3 inflammasome is also of interest to the lab and is being pursued.

The image above is taken from "Ogg1-Dependent DNA Repair Regulates NLRP3 Inflammasome and Prevents Atherosclerosis" showing co-localization of OGG1(red) and mitochondria (green) in an aortic root lesion of a LDLR-/- & High Fat Diet atherosclerosis mouse model. Chlamydia pneumoniae Hijacks a Host Autoregulatory IL-1β Loop to Drive Foam Cell Formation and Accelerate Atherosclerosis
The NLRP3 inflammasome, a multi-component complex that facilitates maturation of IL-1β (a critical proinflammatory cytokine involved in both acute and chronic inflammatory diseases), is activated by many diverse danger signals. The Arditi Lab has found that the mitochondria are a central hub for the various NLRP3 activation signals. NLRP3 activators lead to mitochondrial dysfunction and apoptosis. During this process, mtROS is generated, which results in damaged (oxidized) mtDNA. The oxidized mtDNA is released into the cytosol where it binds to NLRP3 and activates the NLRP3 inflammasome. The lab is now studying the role of Ogg1, an oxidized DNA damage repair gene, in lung and vascular diseases.

Melanoma is a highly immunogenic cancer of the skin that frequently metastasizes to the lung, liver, brain and bone. Once metastasized to a distant site, the five-year survival drops to only 15–20%. In a young population, females have a higher incidence of melanoma; however, by age 80, three times as many males develop the disease. The Arditi Lab is investigating this gender difference with a specific focus on innate immunity and hormonal influences in two syngeneic mouse models of melanoma.

Above shows the increased tumor burden in the lungs of young female C57 mice compared to age-matched male mice.
Scientists in the Arditi Lab are testing healthcare workers to determine whether a decades-old vaccine for tuberculosis, Bacillus Calmette-Guérin (BCG) could provide stopgap protection against COVID-19 until more precise vaccines are available. The BCG vaccine won't prevent people from getting infected with the SARS-CoV-2 virus, but may strengthen the immune system to diminish the effect of infection, resulting in fewer COVID-19 hospitalizations and deaths. The idea was born of researchers' observations that the rates of COVID-19 deaths and serious illness are lower in some developing countries where the BCG vaccine is widely used. Moshe Arditi, MD, leads the BCG vaccine trial, which aims to protect healthcare workers. Arditi at Cedars-Sinai teamed up with investigators at Texas A&M University, MD Anderson Cancer Center and Baylor College of Medicine to recruit 1,800 volunteers at those four centers. The BCG vaccination could make a significant difference in the next year or two until a specific and safe vaccine is developed for COVID-19 and made widely available. Repurposing the tuberculosis vaccine, called TICE® BCG, represents a speedy way to address COVID-19 because the drug has already been proven safe and is approved by the U.S. Food and Drug Administration. In addition to preventing tuberculosis, the vaccine is also used to treat bladder cancer.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes COVID-19, is a coronavirus closely related to SARS-CoV (SARS) and Middle East Respiratory Syndrome (MERS). COVID-19 can manifest in adults as a severe interstitial pneumonia with hyperinflammation, but severe respiratory manifestations are rare in children. Recently, however, multisystem inflammatory system in children (MIS-C) has been recognized in patients that either tested positive for COVID-19 (by PCR or serology) or had epidemiological links to COVID-19.

Discovery of a superantigen-like structure in SARS-CoV-2 that may play a role in the pathogenesis of MIS-C and cytokine storm in adults with severe COVID-19 infection.
After initial reports in the U.K., many cases have now been reported in Europe and New York. MIS-C manifests as persistent fever and hyperinflammation with multi-organ system involvement including cardiac shock, and severe gastrointestinal, renal, hematologic, dermatologic and neurologic symptoms. Although MIS-C was initially described as "Kawasaki disease (KD)-like," it soon became clear that these diseases are different, and that the symptoms and laboratory findings of MIS-C are highly reminiscent of toxic shock syndrome (TSS), rather than KD. Indeed, several recent studies have concluded that MIS-C is a distinct disease from KD and KD shock. The similarities to TSS and the association of MIS-C with COVID-19 indicate that SARS-CoV-2 may possess superantigenic fragments that induce an inflammatory cascade, which may also contribute to the hyperinflammation and cytokine storm features observed in severe adult COVID-19 cases. We therefore hypothesized that SARS-CoV-2 Spike (S) protein may possess superantigenic fragments that could elicit such reactions upon binding proteins involved in the host cytotoxic adaptive immune response.
Using computational biology and bioinformatics, the Arditi Lab and our colleagues at the University of Pittsburgh have discovered a novel superantigen-like structure in the S1 subunit of the SARS-CoV-2 spike protein, which has the structural characteristics to bind to both T cell receptor and major histocompatibility complex class II (https://www.biorxiv.org/content/10.1101/2020.05.21.109272v1). We also discovered that this SARS-CoV-2-specific superantigen-like structure has a remarkable similarity in both sequence and structure to the Staphylococcal enterotoxin B superantigen toxin. These discoveries may unlock the mechanisms that lead to MIS-C as well as to the cytokine storm seen in adults with severe COVID-19 infection. The Arditi Lab is currently working on proving the biological relevance of this newly discovered viral structure.
Contact the Arditi Lab
8700 Beverly Blvd.
Davis Building, Rooms D4024, D4025, D4027
Los Angeles, CA 90048
