Research Areas
The Kaye Laboratory investigates the molecular regulation of T lymphocyte and innate lymphoid cell development and biology. The Kaye Lab has performed pioneering work in this area, and has identified a novel family of four nuclear DNA-binding factors, the founding member of which we designated TOX (thymocyte selection-associated high mobility group box protein). Data from Kaye Lab research has demonstrated that TOX is a critical regulator of aspects of both adaptive and innate immune system development. In addition, TOX plays roles in certain cancers, autoimmunity and CD8 T cell exhaustion. Variants of the gene encoding another family member, TOX3, have been implicated as a risk factor for breast cancer, and, as we have recently uncovered, the protein also plays a key role in brain development. The Kaye Lab is also studying effector cell generation among innate lymphoid cells (ILC), including our recent discovery of a novel ILC2 effector cell subset.
Mechanism of Function of Transcriptional Regulator TOX
TOX is required for thymic development of T cells (so-called positive selection) and development of ILC in the bone marrow. What are the direct gene targets and binding partners of TOX during development of these cell lineages? The Kaye Laboratory is addressing these questions using ChIP-seq and mass spectrometry, along with a new knock-in strain of mouse we created using CRISPR technology that expresses epitope-tagged TOX. The Kaye Lab is also using TOX-reporter mice we created to identify early ILC progenitors and determine the transcriptome of these cells at the single cell level.
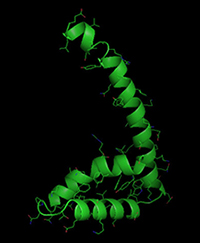
Crystal structure of the TOX DNA-binding domain (Kaye and Murali, unpublished data).
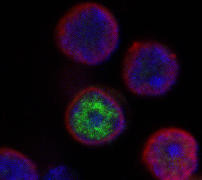
Confocal microscopy image of expression of TOX (green) in the nucleus of a differentiating thymocyte, along with DAPI (blue), and Lamin B (red) to mark the nuclear envelope. TOX is exclusively a nuclear protein excluded from the nucleolus (not shown) and regions of heterochromatin (DAPI bright). (A. Yeckes, unpublished data).
Structure-Function Analyses of the TOX Protein
TOX family members belong to the high mobility group-box superfamily of DNA-binding proteins. The TOX protein is highly evolutionarily conserved in vertebrates. In collaboration with Ramachandran Murali, PhD, in the Murali Laboratory at Cedars-Sinai, we have solved the crystal structure of the DNA-binding domain of TOX (near identical to that of other TOX family members). However, this is only a small part of the protein. In addition, there are two forms of the protein that differ in N-terminal sequence, produced by an interesting mechanism. Using a combination of functional assays, DNA-binding assays and mutagenesis, the Murali and Kaye Labs are exploring the structure-function relationships of the protein as well as its subnuclear organization by super-resolution microscopy.
ILC2 Effector Cell Development
The immune system utilizes a diverse array of cell subtypes that can eradicate pathogens efficiently, while also repressing autoimmunity. Only relatively recently has a diversity of ILC subtypes been identified in mice and humans, some of which have many parallels to CD4+ helper T effector cell subsets, despite their lack of antigen receptors. One subtype, ILC2, has a beneficial role in eradication of parasitic helminths, restoration of lung epithelial barrier function following influenza infection and regulation of beige fat biogenesis. Although ILC2 cells elicit beneficial host responses to pathogens and mucosal damage, these cells are also implicated in disease, most notably allergic responses in the lung. The Kaye Lab has recently reported development of a novel molecularly distinct subpopulation of IL-10 producing ILC2 cells in mouse lung that may be anti-inflammatory. Currently, the Kaye Lab is working to understand the function and molecular regulation of these unique effector cells. In addition, we are studying the production of IL-10 producing human ILC2 cells.
Physiological Role of TOX3 in the Brain and Mammary Gland
The Kaye Lab is addressing the physiological role of TOX3 in the brain and mammary gland using TOX3 conditional knockout mice and various Cre-expressing strains. We recently discovered that loss of TOX3 from neuronal cells leads to a profound specific defect in brain development, which is currently under investigation in collaboration with Joshua Breunig, PhD, in the Breunig Laboratory at Cedars-Sinai.
Other Collaborative Projects
In addition to our work with other laboratories at Cedars-Sinai, the Kaye Laboratory has collaborators at other institutions addressing the role of TOX in the context of CD8 exhaustion, cancer and autoimmunity.
Contact the Kaye Lab
8700 Beverly Blvd.
Davis Building, 5089 (Office), 5094F (Lab)
Los Angeles, CA 90048