Research Areas
The Murine Model of Kawasaki Disease Vasculitis
Kawasaki disease (KD) is an acute febrile illness and systemic vasculitis of unknown etiology that predominantly affects young children, causes coronary artery abnormalities and aneurysms and could potentially result in long-term cardiovascular sequelae and even death. While intravenous immunoglobulin (IVIG) treatment lowers the risk of developing coronary artery aneurysms to 5%, up to 20% of KD patients are IVIG-resistant and are at greater risk for coronary artery aneurysms. Therefore, discovery of more effective treatments to prevent the cardiovascular complications of KD vasculitis is a high priority in pediatric and cardiovascular research. The Noval Rivas Laboratory uses the Lactobacillus casei cell wall extract (LCWE) mouse model of KD vasculitis, which closely reproduces the important histologic as well as pathologic features of the human disease, to further decipher the innate and adaptive immune responses involved in KD development. For more information, see Kawasaki disease: pathophysiology and insights from mouse models.
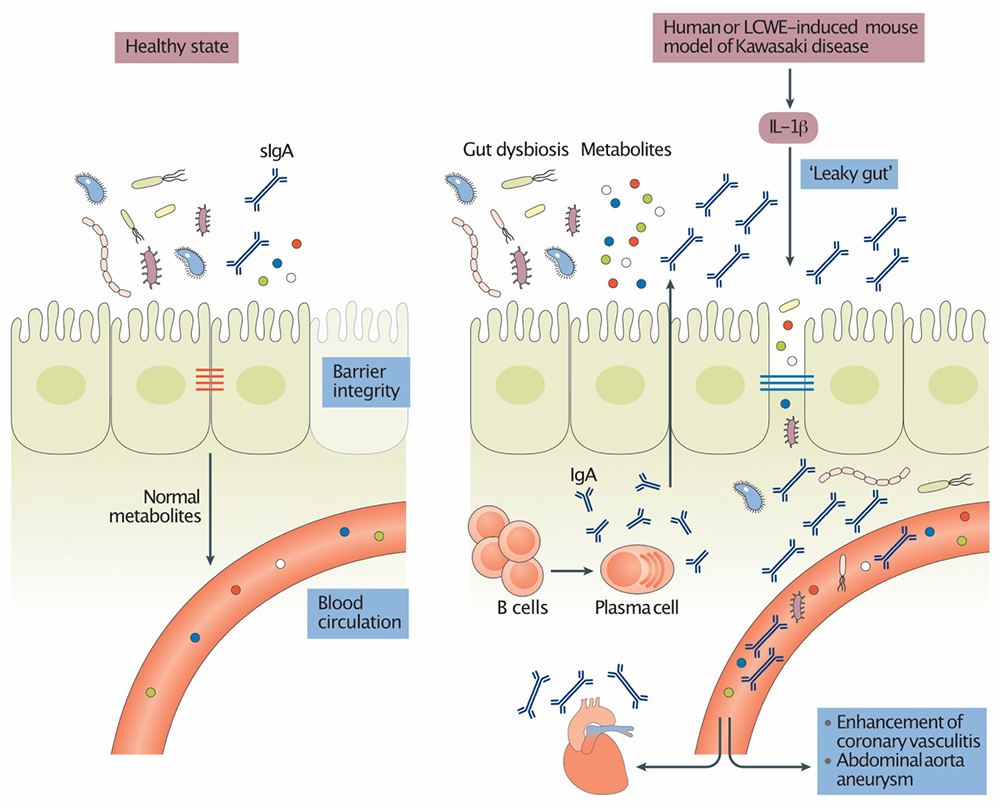
Intestinal Permeability and Gut Microbiome in Kawasaki Disease
The intestinal microbiome is an integral part of our physiology. Intestinal dysbiosis influences the development of a number of immunological and non-immunological disorders, including cardiovascular diseases. The Noval Rivas Laboratory is investigating how the intestinal microbiome influences KD development and pathology. We recently discovered that intestinal permeability and dysregulated mucosal immune responses play a key role in the development of coronary arteritis and aneurysm formation in the LCWE murine model of KD vasculitis. For more information, see Intestinal permeability and IgA provoke immune vasculitis linked to cardiovascular inflammation.
Spatial Transcriptomics and Single-Cell Transcriptional Profiling of Murine Abdominal Aorta Aneurysms
A better understanding and characterization of the cellulome and the molecular networks at steady-state and in cardiovascular lesions that develop during murine KD is essential to understand this pathology and to formulate more precise therapeutic strategies. The Noval Rivas Laboratory is using novel high-throughput sequencing techniques, such as single-cell RNA sequencing and spatial transcriptomics, to characterize the immune cell infiltrations associated with the development of cardiovascular lesions during murine KD. Those novel techniques have the capacities and advantage to assess gene expression in individual cells with high resolution and will highlight cell-type-specific transcriptional networks that underpin disease development.
Autophagy and Mitophagy in Murine Kawasaki Disease Vasculitis
The NLRP3 inflammasome activation and consecutive interleukin-1β (IL-1β) maturation and secretion play a crucial role for the development of both human and murine KD vasculitis. NLRP3 inflammasome can be activated by damaged mitochondrial DNA (mtDNA), which is released after reactive oxidative stress injury and functions as damage-associated molecular patterns. Damaged mitochondria and high levels of mtDNA in the cytosol are recognized by the autophagy machinery, leading to their degradation through the autolysosome. Therefore, autophagy and mitophagy may play an important role in the prevention of cardiovascular lesions in KD vasculitis by diminishing/inhibiting NLRP3 activation, its consecutive IL-1β secretion and the resulting tissue damages. The exact role of autophagy and mitophagy in this context still remains unclear and the lab is currently investigating this pathway.
Intestinal Microbiota Signatures and Immune Responses Underlying Oral Tolerance Breakdown During Food Allergy
The abrupt increase in food allergy incidence implicates environmental factors in disease pathogenesis and correlates with societal and lifestyle changes over the last decades. Allergy to innocuous food antigens reflects a breakdown in oral tolerance, which is enforced by food-allergen specific regulatory T cells (TRegs). However, mechanisms involved in oral tolerance breakdown are still not completely understood. By using Il4raF709 transgenic mice, characterized by enhanced oral allergic sensitization and heightened Th2 immune responses, the Noval Rivas Lab has demonstrated that food allergy is associated with a defective induction of allergen-specific TRegs. Moreover, TRegs from both allergic mice and food allergic patients exhibit decreased suppressive activities due to a Th2-like pathogenic reprograming characterized by elevated GATA3 and IRF4 expression and increased IL-4 production. Food allergic Il4raF709 mice also exhibit a dysbiotic commensal microbiota and oral tolerance can be transferred by fecal transplantation of microbiota from non-allergic mice. We are currently pursuing studies in order to decipher further the host-microbiota interactions involved in food allergy development in the aim to identify fundamental immunological and microbial mechanisms involved in oral tolerance breakdown and food allergy pathogenesis. For more information, please see A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis and Regulatory T cell reprogramming toward a Th2-cell-like lineage impairs oral tolerance and promotes food allergy.
Contact the Noval Rivas Lab
8700 Beverly Blvd.
Davis, Room D4025
Los Angeles, CA 90048