Research Areas
The Omar Lab focuses on developing mechanistic AI models for accurate and early risk assessment in cancer patients using standard clinical data, primarily digitized histopathology slides and radiology scans. Our models are grounded in tumor biology which improves their robustness and interpretability. While our work focuses mainly on prostate cancer, our approaches can be applied to other cancer types as well.
Identifying Digital Pathology Signatures of High-Risk Cancer Phenotypes
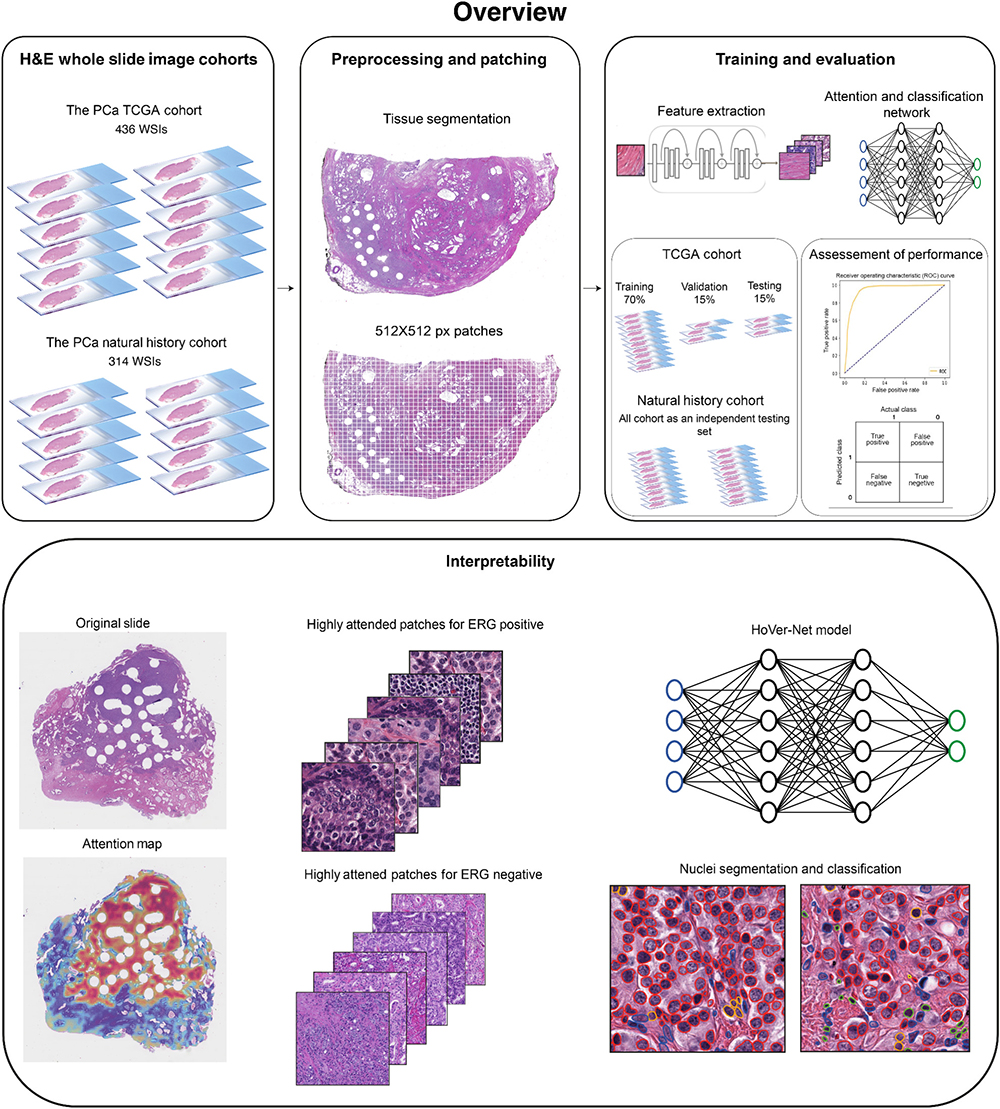
We aim to uncover pathomic signatures from routine H&E-stained whole slide images (WSIs) to inform patients’ prognostication and predict molecular and clinical phenotypes (see Omar et al., Ann Rev of Cancer Bio., 2024). Our work in this domain leverages deep learning algorithms to automate WSIs preprocessing and feature extraction to identify morphometric features associated with certain phenotypes.
For instance, we recently developed a robust model for inferring the status of TMPRSS2:ERG fusion—a key molecular alteration in prostate cancer—from the tissue morphology depicted in routine H&E-stained images of radical prostatectomy specimens (see Omar et al., Mol Cancer Res., 2024).
Deconvoluting the Tumor Microenvironment Composition Across the Spectrum of Cancer Initiation and Progression
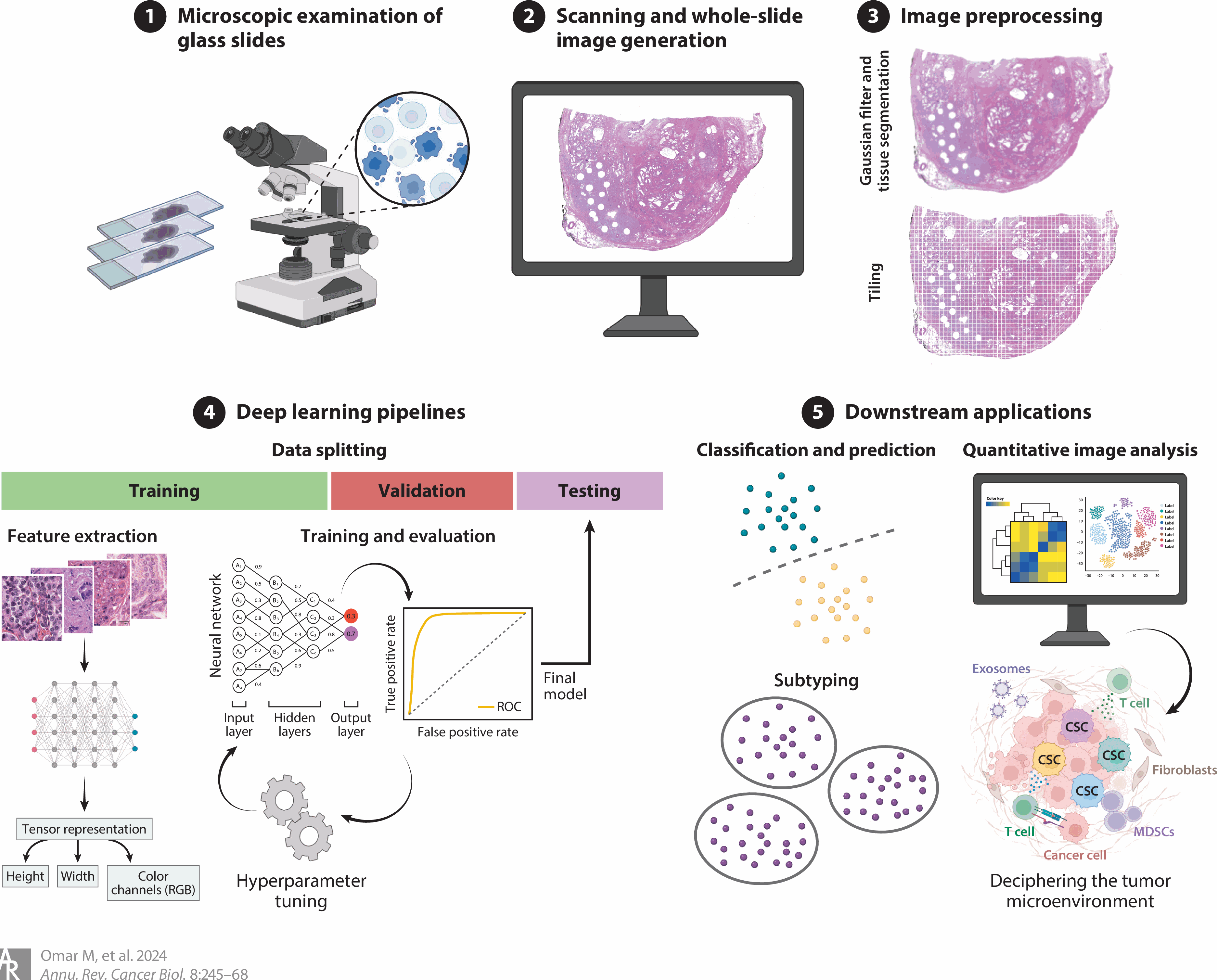
Our lab focuses on developing stage-specific tissue modules that comprehensively capture the complex composition and interplay within the tumor microenvironment at early stages preceding progression. These modules encompass cellular composition, expression profiles, intercellular interactions, gene regulatory networks and spatial neighborhoods. This approach provides an in-depth, systemic understanding of the tumor microenvironment, highlighting the diversity of cell types and the extensive network of molecular and spatial interactions that dictate cellular function and the trajectory of progression.
We employ high-resolution spatial omics to generate single-cell resolution data that recapitulates the spatial distribution and abundance of mRNAs and specific proteins within the tissue context. We leverage this data to construct detailed maps of cellular composition, molecular expression and spatial localization, facilitating a nuanced analysis of how these factors interact within the physical confines of the tumor microenvironment as the tumor progresses.
Developing Multimodal Risk Stratification Tools for Cancer Patients to Assist with Clinical Decision Making
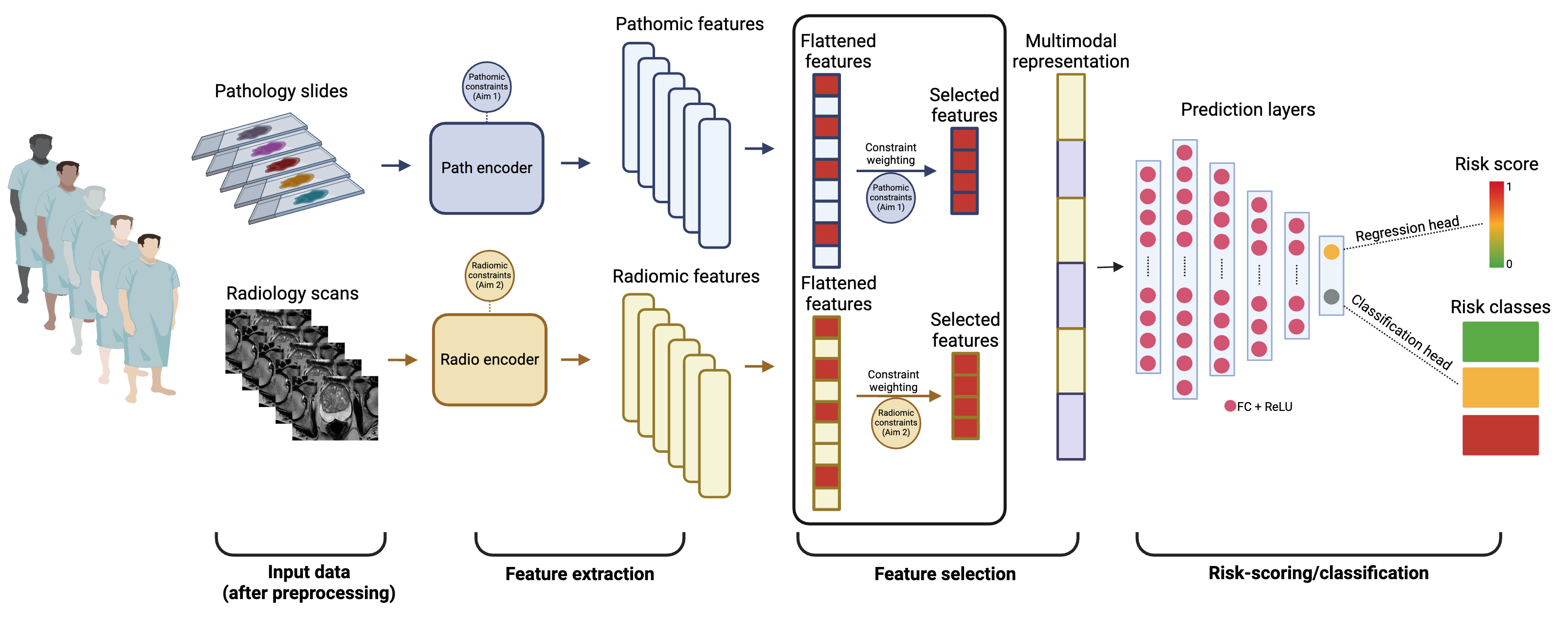
Our lab develops multimodal risk stratification tools that integrate diverse patient-centered data types—including omics, pathomics and radiomics—to enhance risk assessment and inform patient management. Omics offer a deep dive into the molecular underpinnings of tumor progression, while pathomics provide a spatial microscopic view of tumor microenvironment (TME) dynamics by translating pathology images into quantifiable data. Radiomics further enriches this analysis by extracting non-invasive macroscopic features from medical imaging, correlating them with underlying disease mechanisms and outcomes.
The fusion of these data modalities promises to unveil intricate indicators of high-risk disease at very early stages, which cannot be discerned using unimodal approaches.
Contact the Omar Lab
Pacific Design Center
700 N. San Vicente Blvd., Suite G-540F
West Hollywood, CA 90069