Research Areas
Induced Pluripotent Stem Cells (iPSCs) Applied to Disease Modeling and Personalized Therapies
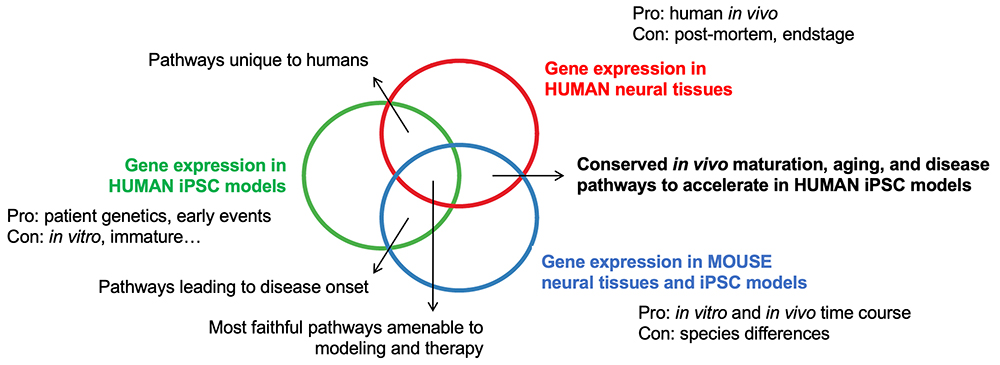
Mapping the Intersection of Tissue and Cell Type-Specific Signatures of Maturation and Aging to Neurodegeneration
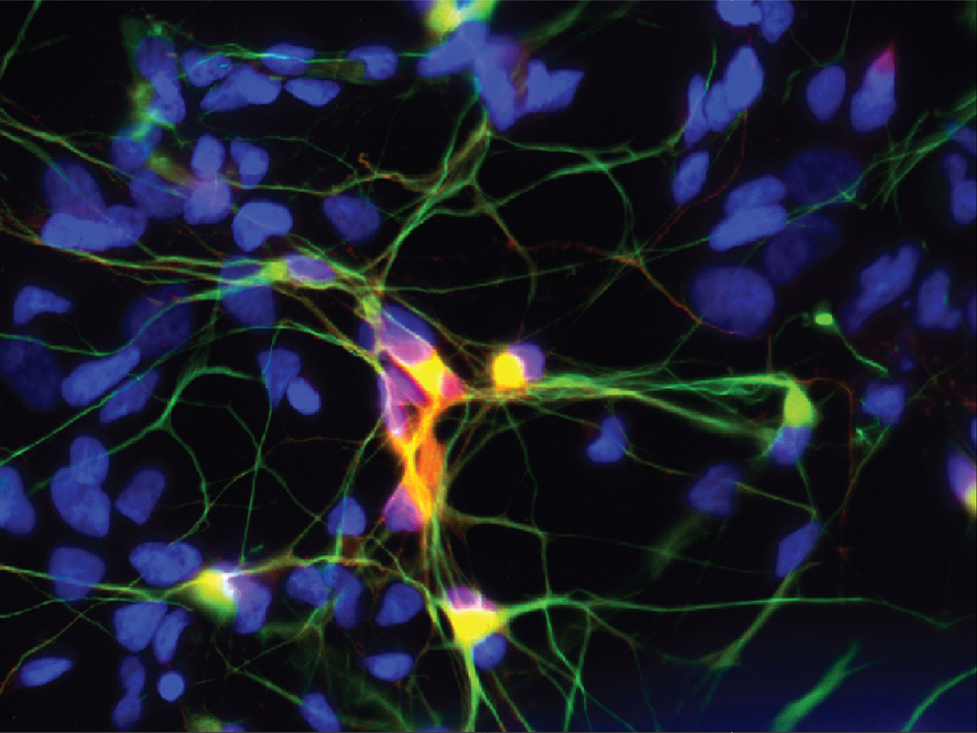
Motor neuron differentiated from human induced pluripotent cells express ChAT (red) and SMI-32 (green).
Can in vitro iPSC models faithfully reflect in vivo development, maturation and aging? We first need to define the molecular signatures of mature and aged tissues and cell types, and which of those signatures are affected in the relevant late-onset diseases that afflict those tissues and cells. Previous work in the Ho Laboratory explored this for amyotrophic lateral sclerosis (ALS). This can establish a roadmap to a target gene expression program, and the Ho Lab can test if our cultures of stem cell derived tissues can traverse that path. If this is achievable, then our stem cell model can assume a physiological state that better reflects the conditions occurring in the adult body, where late-onset diseases manifest.
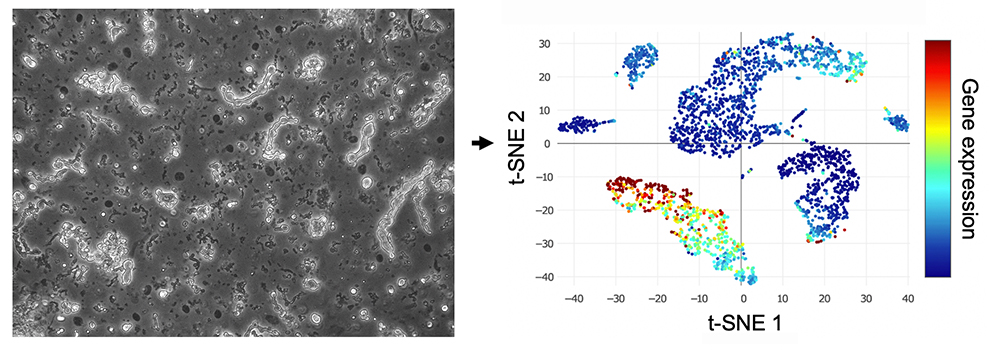
Nuclei extracted form adult neural tissue. (left)
Projection of cellular subtypes based on single-nuclei RNA-seq. (right)
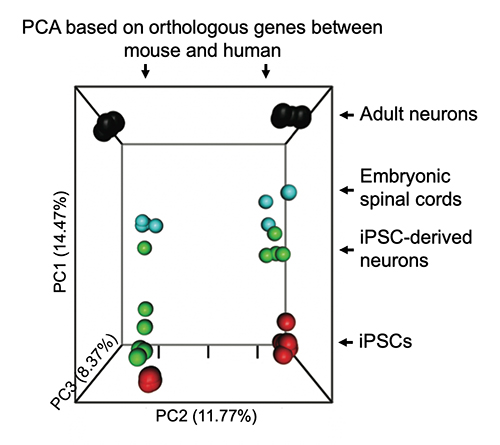
Delineate Gene Expression Networks Conserved or Diverged Across Human and Rodent Species
Animal models have been powerful tools to aid our understanding and treatments of many human diseases, but they are also limited by inherent genetic differences across species. We aim to resolve both the commonalities as well as differences in gene expression programs among widely used mouse models and human systems. These efforts can inform the design of pre-clinical studies and promote successful translation toward clinical pipelines. Also, these investigations will ultimately minimize the number of animals necessary for future research. What is more, as mice enact maturation and aging pathways on a time scale shorter than humans, conserved pathways can potentially be accelerated in human iPSC models.
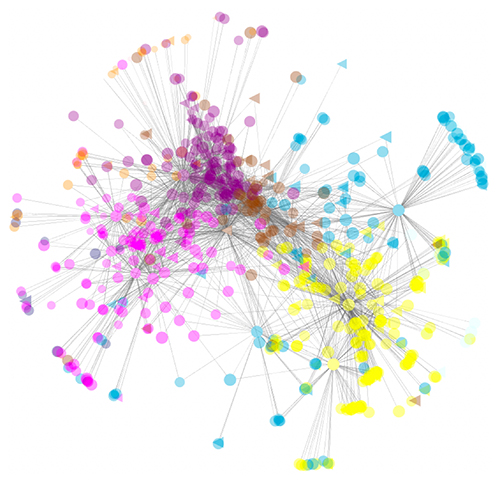
Network graph of gene co-expression modules associated with motor neuron maturation and aging, whihc are also affected in amyotrophic lateral sclerosis.
Engineer Strategies to Accelerate Maturation, Aging and Late-Onset Disease in All Models
The current framework of the Ho Lab is to leverage gene expression programs across three complementary model systems (stem cells, animal and human tissues) to track the origins of late onset disease. The ability to interrogate the fundamental mechanisms that drive development, maturation, aging and disease can help realize our forward vision of personalized, predictive and preventive medicine.