Research Areas
Our lab uses tools, including high-dimensional flow cytometry, single-cell sequencing, multiplex immunohistochemistry, GeoMX and CODEX to study changes in the immune landscape in preclinical and clinical samples following different treatments. Our goal is to find key cells or interactions in the tumor that limit anti-tumor immunity and target them to improve the response to therapy.
Macrophage Regulation of Anti-Tumor Immunity
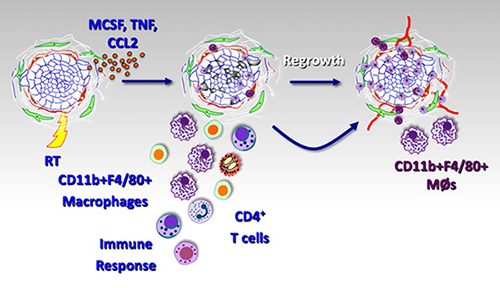
Radiation (RT) and other cytotoxic therapies can elicit cell death that is recognized by the immune system (aka “immunogenic cell death”). This anti-tumor immunity invoked by therapy generates an inflammatory response that consists of innate immune cells (myeloid cells, macrophages, DCs, NK cells) and adaptive immune cells (CD4+ and CD8+ T cells). Numerous studies have demonstrated that this immune response elicited by treatment is critical for optimal therapeutic efficacy. Our lab and others have found that the CD11b+ myeloid-macrophages that appear in tumors following RT work to restrain RT-mediated anti-tumor immunity. Further, RT itself seems to produce a signal that leads to the generation of a suppressive myeloid phenotype following treatment through several different mechanisms. We are currently exploring some of these mechanisms to identify and target the signals that lead to the generation of intratumoral suppressive myeloid cells.
References
- Shiao, S.L. and Coussens, L.M., The tumor-immune microenvironment and response to radiation therapy. J Mammary Gland Biol Neoplasia, 2010. 15(4): p. 411-21.10.1007/s10911-010-9194-9
- DeNardo, D.G., Brennan, D.J., Rexhepaj, E., Ruffell, B., Shiao, S.L., Madden, S.F., Gallagher, W.M., Wadhwani, N., Keil, S.D., Junaid, S.A., Rugo, H.S., Hwang, E.S., Jirstrom, K., West, B.L. and Coussens, L.M., Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov, 2011. 1(1): p. 54-67.10.1158/2159-8274.CD-10-0028
- Shiao, S.L., Ruffell, B., DeNardo, D.G., Faddegon, B.A., Park, C.C. and Coussens, L.M., TH2-Polarized CD4+ T Cells and Macrophages Limit Efficacy of Radiotherapy. Cancer Immunol Res, 2015. 3(5): p. 518-25.10.1158/2326-6066.CIR-14-0232
- Dar, T.B., Henson, R.M. and Shiao, S.L., Targeting Innate Immunity to Enhance the Efficacy of Radiation Therapy. Front Immunol, 2018. 9: p. 3077.10.3389/fimmu.2018.03077
The Microbiome and Radiation-induced Anti-Tumor Immunity
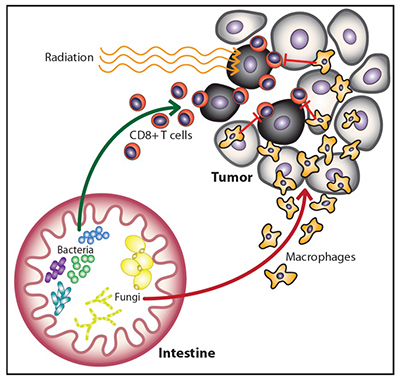
Multiple groups have shown that the bacterial microbiome can influence the response to immunotherapy and chemotherapy. In our recent Cancer Cell paper, our group found that commensal bacteria are similarly necessary for optimal responses to radiation (RT). Surprisingly, we also found that in the absence of bacteria, commensal fungi can and do proliferate. Increasing commensal fungi leads to increasing immunosuppressive activity within the tumor microenvironment. Targeting the commensal fungi enhances the efficacy of RT by reducing suppression of the RT-induced anti-tumor immune response. We are currently exploring the molecular mechanisms underlying the connection between commensal fungi and anti-tumor immunity.
References
- Shiao, S.L., Kershaw, K.M., Limon, J.J., You, S., Yoon, J., Ko, E.Y., Guarnerio, J., Potdar, A.A., McGovern, D.P.B., Bose, S., Dar, T.B., Noe, P., Lee, J., Kubota, Y., Maymi, V.I., Davis, M.J., Henson, R.M., Choi, R.Y., Yang, W., Tang, J., Gargus, M., Prince, A.D., Zumsteg, Z.S. and Underhill, D.M., Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell, 2021. 39(9): p. 1202-1213 e6.10.1016/j.ccell.2021.07.002
- Riquelme, E. and McAllister, F., Bacteria and fungi: The counteracting modulators of immune responses to radiation therapy in cancer. Cancer Cell, 2021. 39(9): p. 1173-1175.10.1016/j.ccell.2021.08.004
Impact of Radiation and Immunotherapy on the Tumor Immune Microenvironment
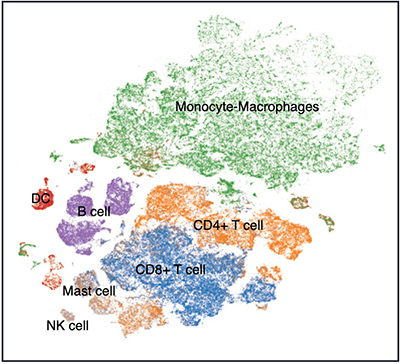
While many groups, including our own, have recognized that radiation can synergize with immunotherapy; unfortunately, clinical results from trials testing the combination have been mixed at best. To better understand the reason why some patients respond to combination therapy while others do not we undertook a phase II trial of focal radiation and pembrolizumab (Anti-PD-1) in triple-negative breast cancer. We collected biopsies pre-treatment, post-Anti-PD-1 and post-Anti-PD-1+RT (8 Gy x 3) (NCT03366844, clinicaltrials.gov) and performed single-cell sequencing and highly-multiplexed immunohistochemistry (CODEX) to understand the phenotypic and spatial changes in the immune landscape following therapy with the help of our bioinformatics partner (Simon Knott, PhD). Current work focuses on the interactions between immune cells and non-immune cells to understand how the non-immune cells shape the tumor immune microenvironment.
References
- McArthur, H., et al. Abstract P3-09-09: Pre-operative pembrolizumab (pembro) with radiation therapy (RT) in patients with operable triple-negative breast cancer (TNBC). Cancer Research 80, P3-09-09-P03-09-09 (2020).
Microglia and Anti-Tumor Neuroimmunity
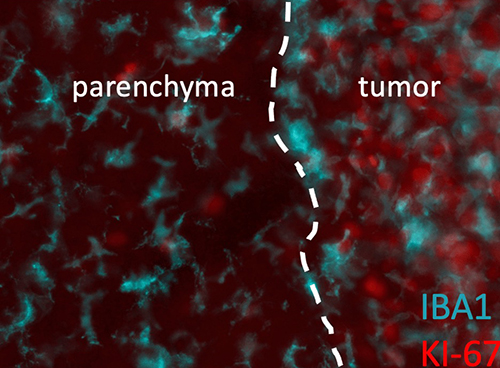
Microglia (Iba1+CD11b+) are the macrophage of the central nervous system and likely serve the same functions as peripheral macrophages, however little is known about their ability to regulate the response to therapy in brain tumors. Immunotherapy to date has been disappointing in the treatment of primary brain tumors with limited responses and we are curious to explore the role of microglia in regulating anti-tumor immunity within the brain. Partnering with the laboratory of Joshua Breunig, we are working on developing unique models of adult gliomas in mice in order to study the impact of microglia on treatment and discover microglia-based targets that can be harnessed to improve the response to standard therapies (radiation and temozolomide) in combination with immunotherapy.
References
- Kim, G.B., et al. Rapid Generation of Somatic Mouse Mosaics with Locus-Specific, Stably Integrated Transgenic Elements. Cell 179, 251-267 e224 (2019).
- Grausam, K. B., et al. Utilizing ‘viral mimicry’ as a novel therapeutic approach in conjunction with anti-PD-1 immunotherapy increases immune activation and extends survival in glioblastoma murine model. AACR Proceedings: Part B (2022)