December 2024 Case
Clinical History
A woman in her 50s with a smoking history presented with an elevated serum creatinine and low-grade proteinuria. She developed COVID-19 4 months before admission, and prior to that her serum creatinine was 0.9 mg/dL. One month before admission she had a sore throat and flank pain and took wormwood extract for 2 weeks before presentation.
The patient has no history of diabetes, hypertension or NSAID use. There is no family history of kidney disease, although her mother has lupus and both patents have hypertension.
Physical exam is unremarkable. Laboratory studies are significant for a serum creatinine of 2.9 mg/dL, hemoglobin of 10.1, 2+ proteinuria, trace hematuria, and no glucosuria. The urine protein/creatinine ratio is 591 mg/g and a 24-hour urine has 1.26 grams protein.
Renal Biopsy Findings
Light microscopy (LM) shows renal cortical inflammation composed of lymphocytes and macrophages with few eosinophils and plasma cells (Fig. 1A). Mature and evolving tubular atrophy and interstitial fibrosis are in much of the cortex, with inflammation in predominantly proximal and atrophied tubules (Fig. 1B). Preserved tubules have epithelial cell acute injury. There is no medullary inflammation. LM of the immunofluorescence (IF) and electron microscopy (EM) specimens shows unremarkable glomeruli (Fig. 2A).
IF studies reveal strong linear staining of tubular basement membranes (TBMs) for IgG, with weaker staining for C3 and both light chains with lambda dominance (Fig. 2B-C). IF for IgG subclasses shows strong IgG1 and weak IgG3 linear staining of TBMs. Indirect immunofluorescence using patient serum on a section of normal human kidney shows similar linear IgG staining of TBMs.
EM shows normal capillary walls with generally preserved podocyte foot processes. No electron dense deposits are identified in glomeruli, TBMs, the interstitium, or vessel walls.
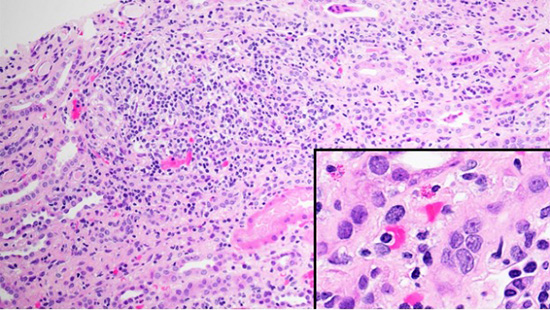
Figure 1A. Light microscopy - (A) H&E stain showing cortical interstitial inflammation composed of lymphocytes and macrophages with few eosinophils and plasma cells (inset).
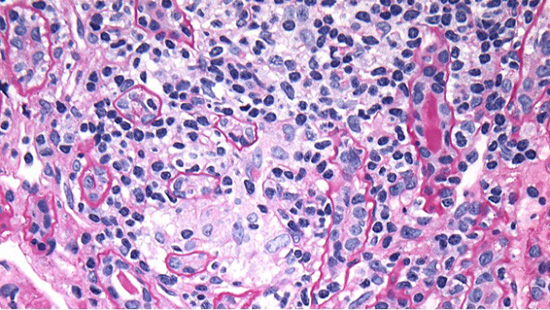
Figure 1B. PAS stain demonstrating atrophic proximal tubules with inflammation (lymphocytes between tubular epithelial cells) and ill-defined granulomatous interstitial inflammation focally, likely secondary to tubular rupture.
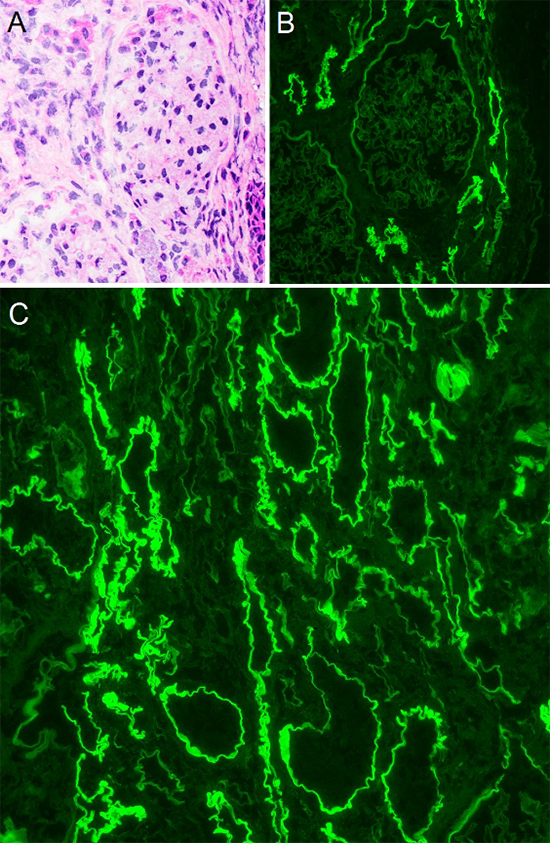
Figure 2: Immunofluorescent findings - (A) H&E stained frozen section showing glomerulus without overt pathologic change. (B) The corresponding field by immunofluorescence shows 4+ linear IgG staining of TBMs with no significant glomerular staining. (C) A separate field with IgG clearly showing 4+ linear staining of TBMs. Note there are tubules which are not staining, likely distal tubules. TBM: tubular basement membrane
Diagnosis
- ANTI-TUBULAR BASEMENT MEMBRANE ANTIBODY DISEASE
- ACUTE TUBULAR INJURY
Discussion
Here we present a case of anti-TBM antibody (aTBM-Abs) disease (anti-TBM disease), characterized by tubulointerstitial inflammation and autoantibodies to a component of the proximal TBM.1 Anti-TBM disease is extremely rare with limited epidemiological data. aTBM-Abs are reported to be found in any age, from older adults to infants from a familial study.2-4 Anti-TBM disease may be primary (idiopathic) or secondary. When primary, the condition is also known as Anti-TBM nephritis. Secondary aTBM-Abs have been reported in the setting of autoimmune disorders, medications, pyelonephritis, and a subset of kidney transplants.5-9 However, aTBM-Abs in secondary forms are of questionable clinical significance, discussed further below.
Patients with anti-TBM disease may present with acute or chronic kidney failure.1 There may be tubular dysfunction with polyuria, polydipsia and/or Fanconi syndrome.10 Urine studies may show microscopic hematuria and/or proteinuria that can be nephrotic range. Kidney biopsy currently is the gold standard for diagnosis as serological tests for aTBM-Abs are not readily available.9 Due to its rarity, patient outcomes have not been well established. Reports are variable, though there are cases with progression to end-stage kidney disease (ESKD).4,11
The histopathology of anti-TBM disease shows interstitial inflammation predominantly composed of lymphocytes and plasma cells, though eosinophils or neutrophils can be present. As the pathogenic antigen ("TIN-ag", discussed later) is expressed in proximal tubules, inflammation is concentrated around proximal tubules with associated tubulitis and acute tubular injury. The interstitium may be edematous and tubular inflammation can lead to TBM rupture, as seen in this case though these are non-specific findings. Glomerular and vascular compartments show no specific changes unless there is another superimposed disease. Crescents suggest the co-occurrence of anti-glomerular basement membrane (GBM) antibodies. The linear staining of proximal TBMs for IgG is highly characteristic and required for a diagnosis. Complement proteins are also often present, and may show variability in intensity, extent, and linearity. Electron microscopy shows no TBM deposits or other specific findings.
The exact patho-etiology of anti-TBM disease is uncertain, but several studies have identified likely antigenic targets. In anti-TBM disease, a non-collagenous 54 to 58-kD protein has been most consistently identified, referred to as tubulointerstitial nephritis antigen (TIN-ag).4,12,13 This protein is Involved in tubulogenesis and interacts with other structural proteins of the TBM extracellular matrix, such as collagen, laminin, and integrins.13 aTBM-Abs bound to proteins of other sizes have been isolated, though it is thought that some of these represent TIN-ag bound to other proteins or aggregates of TIN-ag.11,12 Drug-associated anti-TBM disease is thought to be due to proximal tubular reabsorption and concentration of drugs which leads to covalent bonding between drugs and TBM proteins, with subsequent formation of aTBM-Abs to the hapten-protein conjugate.6,14 Drugs reported in anti-TBM disease include methicillin and ciprofloxacin6,15 Some reported drug associations may be better classified as a tubulointerstitial nephritis with pseudo-linear TBM staining due to TBM immune-complex deposition.16 Infections may trigger aTBM-Ab formation via parenchymal damage and autoimmunization to typically sequestered self-antigens, such as TIN-ag.7,9 Anti-TBM disease has been reported de novo in transplant kidneys.5 This is presumed to be due to recognition of donor TBM antigens not present in the recipient.5,17 Notably, aTBM-Abs that form with secondary associations may not necessarily be pathologic. Limited cases have reported antibody resolution, either spontaneously or following treatment, with no long-term impact on renal or graft function.10,17,18
Linear IgG staining is the defining biopsy feature, but similar immunofluorescent findings may be seen in other clinical conditions. Diabetic nephropathy (DN) is a common biopsy finding with pseudolinear staining of TBMs with IgG.10 The distinction is usually not difficult since DN also shows staining of GBMs, and with equivalent staining of TBM and GBM for albumin. Several autoimmune diseases may show extraglomerular immune complex deposition including along TBMs. The pattern is usually granular but can mimic linear staining. Anti-brush border antibody tubulointerstitial nephritis can also show widespread staining of TBM, though the pattern is typically still discernable as granular and electron microscopy will demonstrate electron dense deposits along TBMs. It's stated that "aTBM-Abs" are found in 50-70% of patients with anti-GBM antibody disease.9,19 While some cases have isolated aTBM-Ab, some cases likely represent antibody cross-reactivity to collagen in TBMs.20 By LM alone, tubulointerstitial nephritis can elicit a wide differential of the underlying pathogenesis.
In conclusion, we present a case of anti-TBM disease, a rare finding. In our case, it is possible, though indeterminate, that COVID-19 triggered the autoantibody response. Unusually, our case showed somewhat restriction for IgG1-lambda; anti-TBM disease typically is polyclonal. The significance of this is unknown. One recent case of anti-TBM disease was reported in a patient with chronic lymphocytic leukemia.21 Our patient did not have a known history of hematolymphoid neoplasm. The patient was treated with rituximab and had improvement in her serum creatinine. Follow-up testing using indirect immunofluorescence with patient serum is planned to determine if the antibody has disappeared. Due to the association with systemic diseases and reported progression to ESKD, patients with anti-TBM disease should be followed closely.
References
- Lusco MA, Fogo AB, Najafian B, Alpers CE. AJKD Atlas of Renal Pathology: Anti-Tubular Basement Membrane Antibody Disease. Am J Kidney Dis. Jul 2017;70(1):e3-e4. doi:10.1053/j.ajkd.2017.05.001
- Cattran DC. Circulating anti-tubular basement membrane antibody in a variety of human renal diseases: detection and significance. Nephron. 1980;26(1):13-9. doi:10.1159/000181943
- Tay AH, Ren EC, Murugasu B, et al. Membranous nephropathy with anti-tubular basement membrane antibody may be X-linked. Pediatr Nephrol. Aug 2000;14(8-9):747-53. doi:10.1007/pl00013429
- Iványi B, Haszon I, Endreffy E, et al. Childhood membranous nephropathy, circulating antibodies to the 58-kD TIN antigen, and anti-tubular basement membrane nephritis: an 11-year follow-up. Am J Kidney Dis. Dec 1998;32(6):1068-74. doi:10.1016/s0272-6386(98)70085-x
- Klassen J, Kano K, Milgrom F, et al. Tubular lesions produced by autoantibodies to tubular basement membrane in human renal allografts. Int Arch Allergy Appl Immunol. 1973;45(5):675-89. doi:10.1159/000231067
- Border WA, Lehman DH, Egan JD, Sass HJ, Glode JE, Wilson CB. Antitubular basement-membrane antibodies in methicillin-associated interstitial nephritis. N Engl J Med. Aug 22 1974;291(8):381-4. doi:10.1056/nejm197408222910803
- Lindqvist B, Lundberg L, Wieslander J. The prevalence of circulating anti-tubular basement membrane-antibody in renal diseases, and clinical observations. Clin Nephrol. Apr 1994;41(4):199-204.
- Dixit MP, Scott KM, Bracamonte E, et al. Kimura disease with advanced renal damage with anti-tubular basement membrane antibody. Pediatr Nephrol. Dec 2004;19(12):1404-7. doi:10.1007/s00467-004-1593-y
- Rifai AO, Denig KM, Caza T, et al. Antitubular Basement Membrane Antibody Disease Associated with Nivolumab Infusion and Concomitant Acute Pyelonephritis Leading to Acute Kidney Injury : a Case Report and Literature Review. Case Rep Nephrol. 2023;2023:6681756. doi:10.1155/2023/6681756
- Jennette JC, D'Agati V. Heptinstall's Pathology of the Kidney. 8 ed. Lippincott Williams & Wilkins; 2023:chap Noninfectious Tubulointerstitial Nephritis.
- Katz A, Fish AJ, Santamaria P, Nevins TE, Kim Y, Butkowski RJ. Role of antibodies to tubulointerstitial nephritis antigen in human anti-tubular basement membrane nephritis associated with membranous nephropathy. Am J Med. Dec 1992;93(6):691-8. doi:10.1016/0002-9343(92)90205-p
- Butkowski RJ, Kleppel MM, Katz A, Michael AF, Fish AJ. Distribution of tubulointerstitial nephritis antigen and evidence for multiple forms. Kidney Int. Nov 1991;40(5):838-46. doi:10.1038/ki.1991.283
- Ikeda M, Takemura T, Hino S, Yoshioka K. Molecular cloning, expression, and chromosomal localization of a human tubulointerstitial nephritis antigen. Biochem Biophys Res Commun. Feb 5 2000;268(1):225-30. doi:10.1006/bbrc.2000.2103
- Perazella MA, Markowitz GS. Drug-induced acute interstitial nephritis. Nat Rev Nephrol. Aug 2010;6(8):461-70. doi:10.1038/nrneph.2010.71
- Ortiz A, Plaza JJ, Egido J. Ciprofloxacin-associated tubulointerstitial nephritis with linear tubular basement membrane deposits. Nephron. 1992;60(2):248. doi:10.1159/000186754
- Gabow PA, Lacher JW, Neff TA. Tubulointerstitial and glomerular nephritis associated with rifampin. Report of a case. Jama. Jun 7 1976;235(23k0):2517-8. doi:10.1001/jama.235.23.2517
- Rotellar C, Noel LH, Droz D, Kreis H, Berger J. Role of antibodies directed against tubular basement membranes in human renal transplantation. Am J Kidney Dis. Feb 1986;7(2):157-61. doi:10.1016/s0272-6386(86)80138-x
- Makker SP, Widstrom R, Huang J. Membranous nephropathy, interstitial nephritis, and Fanconi syndrome-- glomerular antigen. Pediatr Nephrol. Feb 1996;10(1):7-13. doi:10.1007/bf00863427
- McAdoo SP, Pusey CD. Anti-Glomerular Basement Membrane Disease. Clin J Am Soc Nephrol. Jul 7 2017;12(7):1162-1172. doi:10.2215/cjn.01380217
- Audard V, Hellmark T, El Karoui K, et al. A 59-kD renal antigen as a new target for rapidly progressive glomerulonephritis. Am J Kidney Dis. May 2007;49(5):710-6. doi:10.1053/j.ajkd.2007.02.002
- Ebrahimi N, Najafian B, Chen Wongworawat Y, Norouzi S, Abdipour A. Anti-Tubular Basement Membrane Antibody Nephritis Manifesting in a Patient With Chronic Lymphocytic Leukemia: A Very Rare Case Report. Journal of Investigative Medicine High Impact Case Reports. 2024;12:23247096241281612. doi:10.1177/23247096241281612
